Research interests
(1) Signal integration at the cellular level and its implications in breast cancer:
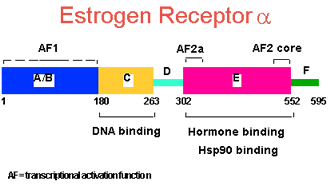
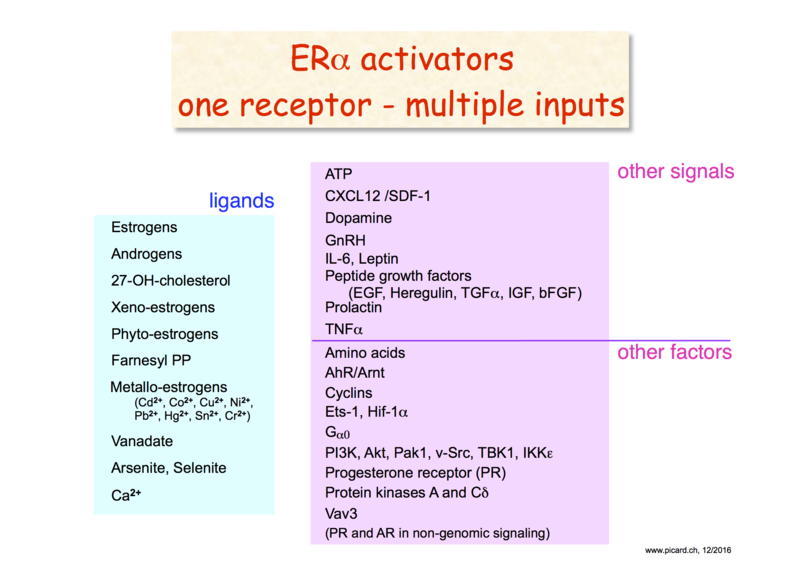
Steroid receptors such as the estrogen receptors are intracellular soluble receptors. Their activities as transcription factors are regulated by ligand binding. In addition to being regulated by their cognate ligands, for example estrogen in the case of the estrogen receptors, they also integrate other extracellular and intracellular signals. They can be activated by a wide spectrum of direct ligands and factors that act through other signaling pathways.
Since estrogens stimulate the proliferation of a large proportion of breast cancers that express the estrogen receptor α (ER), endocrine therapy aims to block the production of estrogens or access of estrogens to the receptor. However, signaling crosstalk can lead to the activation of ER even in the absence of estrogens or in the presence of the commonly used anti-estrogen tamoxifen.
Our goals were to understand: (i) the molecular mechanisms of signal transduction by ER and its crosstalk with other intra- and extracellular signals; (ii) the genomics of signaling crosstalk; (iii) the ER interactors, both genetic and biochemical, cytoplasmic and nuclear, that shape its responses; (iv) what factors contribute to rendering the ER tamoxifen-resistant; (v) the physiological and pathological implications of these mechanisms, notably for breast cancer progression and resistance to tamoxifen; (vi) the effects of alternate cellular states of breast cancer cells on ER responses (for example, of cells cultured in 3D or upon epithelial-mesenchymal transformation).
(2) Molecular matchmakers: The Hsp90 molecular chaperone machine:
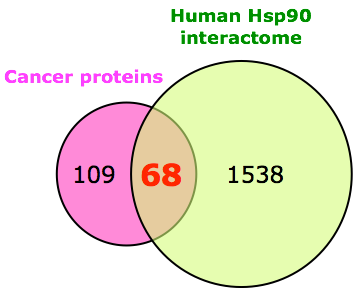
Cellular “matchmakers” such as the molecular chaperone heat-shock protein 90 (Hsp90) and its partner proteins are required for a multitude of cellular processes and for the maintenance of cellular protein homeostasis. The ATP-dependent Hsp90 machine assists a subset of proteins during posttranslational folding and macromolecular assembly, and in some cases it even plays a regulatory role. In eukaryotes, this molecular machine is essential for viability. Cancer cells and a large proportion of cancer driver genes are particularly Hsp90-dependent and sensitive to Hsp90 inhibitors. Although this differential sensitivity is still poorly understood, it has led to major efforts to develop Hsp90 inhibitors for anti-cancer therapy.
We were particularly interested in determining (i) the in vivo functions of the Hsp90 molecular chaperone complex both in mammalian cells and in the mouse, using genetic, biochemical, proteomic and pharmacological tools; (ii) the specific functions of key components such as the two core components Hsp90α and Hsp90β, and the co-chaperones p23, Aarsd1, Stip1 (Hop), Aha1 and Aha2; (iii) the key functions of Hsp90 as a network hub in the human proteome; (iv) the mechanisms responsible for the differential sensitivity to Hsp90 inhibitors of cancer cells versus normal cells; (v) the functions of the mitochondrial Hsp90 isoform Trap1 in the mouse and in cells.


